If you have relapsing multiple sclerosis (MS) and your current medication isn’t keeping the disease under control, your neurologist might discuss Lemtrada (alemtuzumab) as a possible option. This is not a first-line treatment — it’s generally reserved for people whose MS remains active despite trying other medications — but for some, it can significantly reduce relapses and slow disability progression.
Why Lemtrada Is Used
MS is an autoimmune disease in which the immune system attacks myelin, the protective coating around nerves in the brain and spinal cord. Even with treatment, some people continue to have relapses, new MRI lesions, or gradual disability increase. Lemtrada was developed for these patients.
Lemtrada targets a protein called CD52 found on the surface of certain immune cells (T and B lymphocytes). When it binds to CD52, most of these cells are removed from the blood. The immune system then gradually rebuilds, often with fewer of the aggressive cells thought to drive MS attacks. This “immune reset” can help reduce inflammatory activity in the central nervous system.
How Effective Is It?
In large clinical trials (CARE-MS I and CARE-MS II), Lemtrada was compared to interferon beta-1a, a commonly used injectable MS medication. Lemtrada significantly reduced annual relapse rates and, in some patients, slowed the build-up of disability.
Many people in these studies only needed two annual treatment courses to maintain disease control for several years. Long-term follow-up and real-world data from MS clinics also show sustained disease stability in many patients, although some require additional courses.
How It’s Given
Lemtrada is delivered by intravenous (IV) infusion in two main courses:
- First course: 5 consecutive daily infusions
- Second course: 3 consecutive daily infusions, 12 months later
If the disease becomes active again, additional 3-day courses may be given, but never sooner than one year after the previous course.
Because of the risk for serious side effects, Lemtrada can only be given at certified infusion centers by trained staff.
Safety Risks
Lemtrada is a powerful therapy and carries significant risks. Serious side effects may include:
- Autoimmune conditions
– Thyroid disease (occurs in up to 40% of patients)
– Immune thrombocytopenia (low platelets)
– Kidney inflammation (anti-GBM disease) - Infusion reactions
– Rash, headache, fever, nausea, chest discomfort, or more severe allergic responses - Stroke or arterial tear
– Reported mostly within 3 days of infusion - Certain cancers
– Thyroid cancer, melanoma, lymphoproliferative disorders - Infections
– Increased risk due to reduced immune cell counts; Listeria, herpes viruses, and rare brain infections like PML have been reported
Because of these risks, Lemtrada is only available through a Risk Evaluation and Mitigation Strategy (REMS) program in the U.S.
The REMS Program
If you and your neurologist decide to proceed with Lemtrada, you must enroll in the LEMTRADA REMS Program, which requires:
- Certified prescriber and infusion site
- Monthly blood and urine tests for 4 years after your last infusion
- Thyroid tests every 3 months
- Annual skin checks for melanoma
Monitoring may be extended beyond 4 years if abnormal results or symptoms are found. Even if you feel fine, these tests are essential for catching problems early.
Preparing for Treatment
Before your first infusion, your care team will:
- Perform baseline blood and urine tests
- Check your skin for suspicious moles or lesions
- Review your vaccination history and give needed vaccines at least 6 weeks before treatment
- Provide food safety instructions to avoid Listeria-containing foods
If you are of childbearing potential, you will need to use effective birth control during treatment and for 4 months afterward.
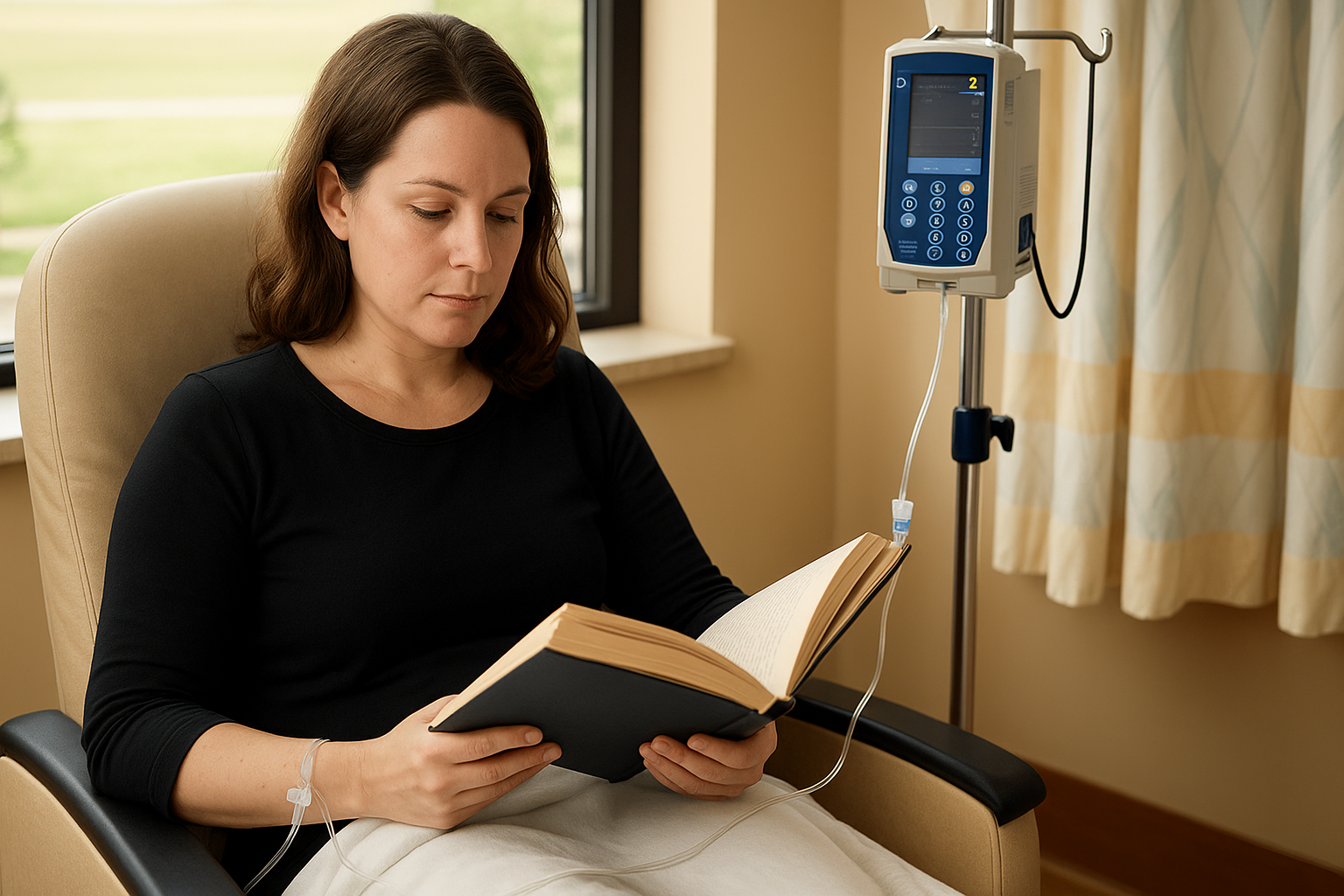
During Treatment
Each infusion lasts at least 4 hours, followed by at least 2 hours of observation. On the first 3 days of each course, you will receive steroids to help prevent infusion reactions, and additional medicines for fever or allergic symptoms if needed.
You can read, watch TV, or rest during the infusion, but staff will check on you frequently for any reaction signs.
Recovery and Self-Care
Fatigue is common after infusions and can last several days or longer. Plan for:
- Extra rest and light activity only
- Having help at home if needed
- Keeping a record of any symptoms to review with your neurologist
Avoid pushing yourself too soon. Gradually return to normal routines once you feel ready.
Cost and Support
Lemtrada is an expensive medication, but the manufacturer offers a co-pay program and other support options. Your MS care team or a case manager can help check your insurance coverage and assist with applications for financial aid.
Lemtrada FAQs
1. Who should consider Lemtrada?
Adults with relapsing forms of MS whose disease remains active after at least two other MS treatments. Not for clinically isolated syndrome (CIS) or progressive forms of MS.
2. How does Lemtrada work?
It removes most circulating T and B lymphocytes, reducing immune system attacks on myelin. Over time, immune cells repopulate in a way that may be less likely to cause MS relapses.
3. How is Lemtrada given?
- Year 1: 5 daily infusions
- Year 2: 3 daily infusions, 12 months later
Additional 3-day courses may be given later if necessary.
4. What are the main safety concerns?
Autoimmune diseases, infusion reactions, stroke/arterial tear, certain cancers, and infections. Requires ongoing monitoring.
5. What monitoring is required?
Monthly blood and urine tests for 4 years after last infusion, thyroid tests every 3 months, and yearly skin checks. Monitoring may be extended.
6. How should I prepare before starting?
Baseline lab tests, skin check, vaccinations if needed, and food safety precautions. Birth control during treatment and for 4 months afterward.
7. What is the infusion process like?
At least 4 hours for infusion plus 2 hours observation. Steroids given for first 3 days of each course. Staff monitor closely for reactions.
8. How should I plan for recovery?
Expect possible fatigue for several days. Plan for rest, light activities, and symptom tracking.
9. How much does Lemtrada cost?
High cost, but assistance programs exist. Your MS team can help you apply for co-pay or other aid programs.
10. What should I ask my neurologist?
- What alternatives do I have?
- How will Lemtrada fit into my long-term MS plan?
- What immediate side effects should I watch for?
- How will my monitoring be organized?
- Who do I contact for new symptoms?
Bottom line: Lemtrada can be an effective option for people with relapsing MS who have not responded to other treatments. However, it requires strict safety monitoring and a commitment to follow-up care. Discuss the benefits and risks thoroughly with your neurologist before making a decision.